Researchers at the University of Michigan Health Rogel Cancer Center are one step closer to understanding how pediatric DIPG tumors work.
Diffuse intrinsic pontine glioma, or DIPG, is the most aggressive pediatric brain tumor and incredibly difficult to treat since surgery isn’t feasible and recurrence is likely after radiation.
These tumors are often defined by the H3K27M driver histone mutation. Rogel researchers, led by Daniel Wahl, M.D., Ph.D., wanted to understand how this mutation affects DIPG tumor metabolism and influences radiation resistance. “These questions pointed us straight to purine metabolism,” said Wahl, associate professor of radiation oncology and neurosurgery.
The findings were published in Cancer & Metabolism.
Wahl’s team has previously explored how purines are metabolized in adult glioblastoma, including a clinical trial to see how blocking purine metabolism improves treatment success.
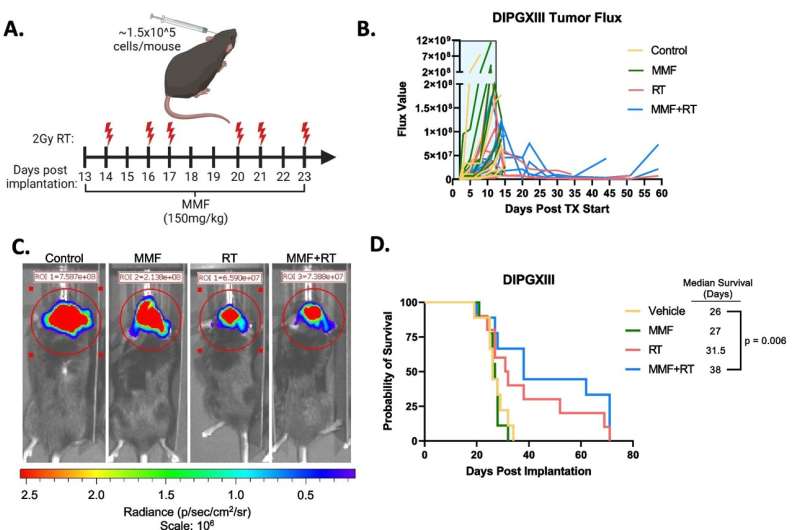
Here, the team tried a similar method of inhibiting purine synthesis. While it worked well in DIPG cells, it was less impressive in animal models.
Wahl explains that, unlike many adult glioblastomas, DIPG tumors seem to rely on two different routes to make purines. He likens it to a road with two on-ramps and says, “In adult GBMs, one of these on-ramps seems to be blocked, almost like it’s under construction. If you block the second on-ramp with a drug, it’s a real problem for the tumor. But in these pediatric brain tumors, both on-ramps appear to be wide open. So a drug that just blocks one of them doesn’t really slow down the DIPGs.”
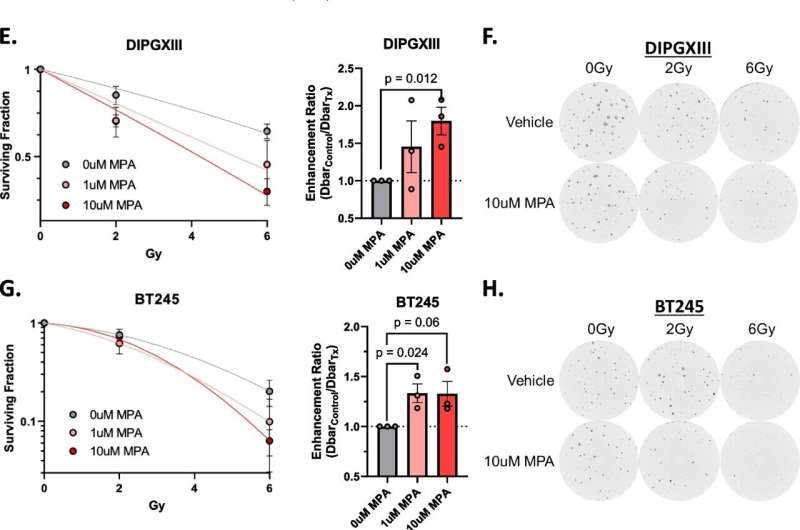
Although there isn’t a drug available to block this “second” on-ramp, Wahl was able, through genetic silencing, to stop purines from being made inside the cell. “When we did that, radiation worked great,” Wahl said.
Erik Peterson, lead author and cancer biology graduate student agrees: “A better understanding of how these tumors evade treatment means that we’re better equipped to develop new strategies to beat them at their own game and improve the outlook for kids with this disease.”
Next, the researchers want to build on these findings to better understand why pediatric DIPG tumors rely on a different route from adult tumors, and how they can develop therapies to block it. With this additional work, the research team is hopeful that they could make a dent in this disease for patients in the future.
More information:
Erik R. Peterson et al, Purine salvage promotes treatment resistance in H3K27M-mutant diffuse midline glioma, Cancer & Metabolism (2024). DOI: 10.1186/s40170-024-00341-7
Citation:
Study finds pediatric brain tumors rely on different metabolic ‘route’ to fuel treatment resistance (2024, June 13)
retrieved 13 June 2024
from https://medicalxpress.com/news/2024-06-pediatric-brain-tumors-metabolic-route.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.